In today’s ‘selfie-obsessed’ culture, there is no other anatomical region which carries greater social prestige and importance, than a well-proportioned ‘youthful’ face. In particular, the youthful midface has been described in the literature as a “single convexity in harmony with the lower eyelid / cheek junction with superiorly positioned voluminous malar fat pads and concavity in the buccal regions” [1].
Undoubtedly, a major development in aesthetic facial surgery has been the recognition that facial volume loss, irrespective of cause (physiological ageing or pathological disease), markedly affects the three-dimensional (3D) topography of the face. Changes to the structural framework of the craniofacial skeleton are well recognised to be a consistent key contributor affecting the draping of the overlying facial musculature and soft tissue envelope, particularly apparent within the midfacial region [2,3,4]. Volume depletion has also been cited as a ‘major’ contributor to age-related changes within the skin, such as superficial lines and / or wrinkles [5]. Midfacial volume loss, whether from diminishing bony support or loss of soft tissue, must be corrected in order to restore a youthful appearance [5].
Fundamentally, the aim of volume restoration is to target all levels of the facial soft tissue envelope commencing with re-establishment of deep structural support. Midfacial rejuvenation techniques are now targeted towards providing underlying structural support and are focused on replenishing ‘volume loss’ in the deeper tissues such as fat, muscle and bone. There are a wide variety of procedures available to address midfacial volume loss and augmentation including injectable fillers, autologous fat transfer, lifting procedures with facial threads or traditional surgery and the use of alloplastic facial implants. The use of midfacial ‘malar’ implants is the second most frequently used alloplastic implant in facial cosmetic surgery after chin alloplastic augmentation [6]. Although a surgical procedure, in our opinion the popularity of malar implants is associated with a number of advantages including: 1) their permanent nature, 2) ease of placement, 3) resilience to ‘physiological ageing’, 4) range of implant choice and material, 5) low complication rate, and 6) high patient satisfaction.
Implant types
Over the past decade, there has been considerable refinement in the form and placement of malar implants. Malar implants may either be prefabricated and trimmed to size perioperatively to conform to the underlying anatomy or custom-made. In our practice, custom-made malar implants fabricated by computer-assisted design / computer-assisted manufacturing technology are used for challenging cases of facial deformity to camouflage / aesthetically correct pronounced skeletal deficiencies and / or asymmetries.

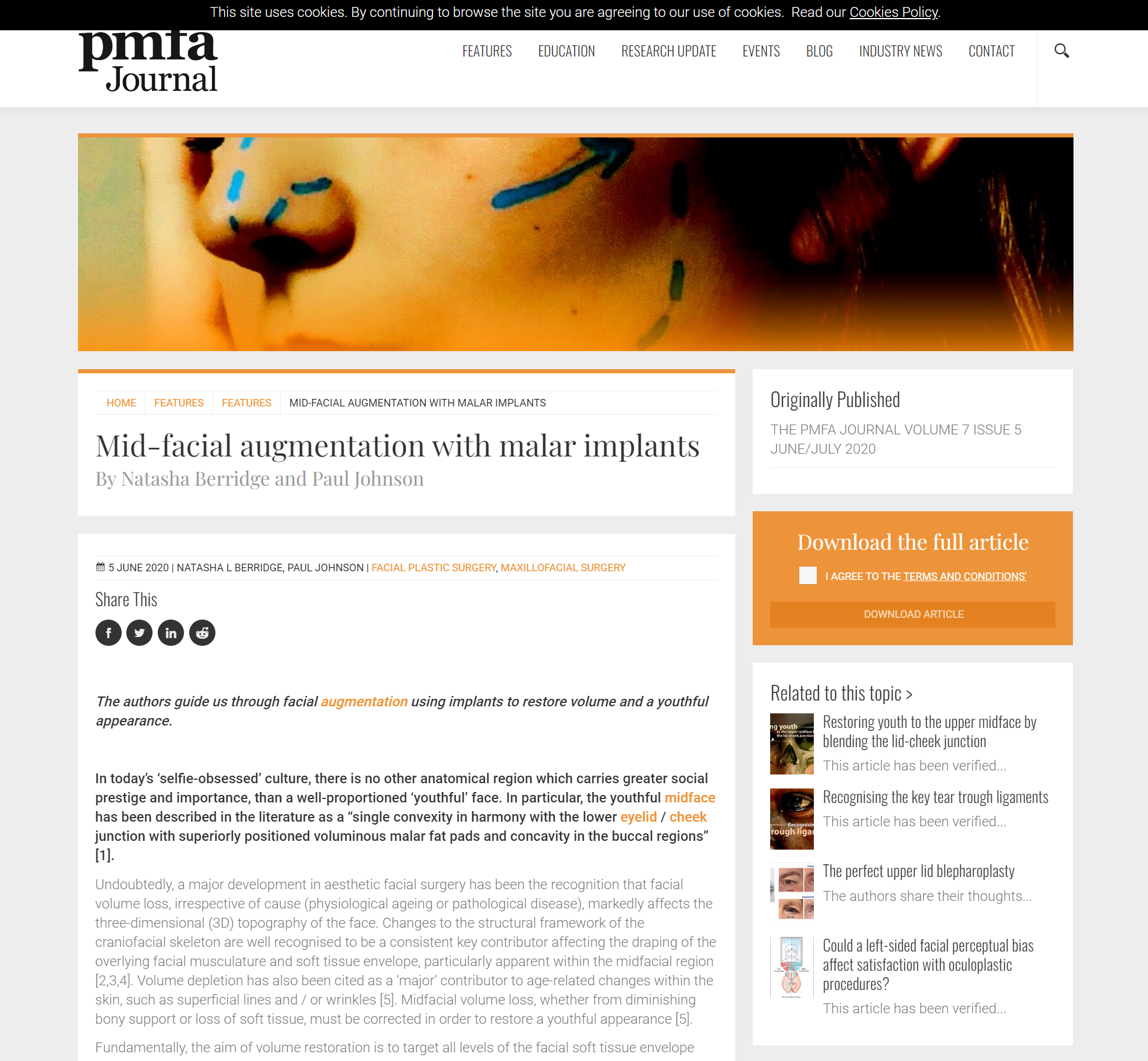
Figure 1: A prefabricated Medpore malar implant.
Commonly used implant materials have included silicone rubber, porous polyethylene, polymethylmethacrylate, polyetheretherketone (PEEK) [6,7] and titanium (Figure 1). It is the second author’s preference to use Medpore (Stryker) as it comes in a range of sizes (small, medium and large) and can be easily trimmed to allow ease of placement and a good anatomical fit. Additionally, Medpore implants have a porosity between 125-250 microns, which allows for fibrous tissue ingrowth and therefore minimises the implant’s tendency to migrate or erode through the underlying bone. The porosity of Medpore malar implants allows overlying soft tissue ingrowth which can potentially lead to a more difficult implant removal compared to the smooth surface implants such as silicone or titanium should the implant need to be extracted. Significant soft tissue injury and defects can occur with removal, as well as fragmentation of the implant [7].
Patient assessment
A comprehensive patient assessment is key to the success of correcting midfacial volumetric and contour deficiencies. An integral part of this fundamental process is to fully educate the patient regarding the aetiology of midfacial volume loss, the level of the facial envelope and / or deep structural support affected, and the best available treatment options to achieve the desired end result. It is crucial to remain mindful that volume restoration in one facial region may inadvertently exert an aesthetic effect, deemed to be either positive or negative in an adjacent facial area.
“A comprehensive patient assessment is key to the success of correcting midfacial volumetric and contour deficiencies”
In our practice, the most common indication for malar implant placement is midfacial / maxillary hypoplasia. Clinically, patients with this facial skeletal deformity will exhibit the classical stigmata of deficient midfacial soft tissue envelope, prominent nasolabial folds, ‘negative vector’ relationship of the globe relative to the deficient infraorbital rim, acute nasolabial angles and a convex facial profile (Figure 2).

Figure 2: Frontal, oblique and lateral views highlighting the classical gaunt and
hollow midfacial profile with of a patient with maxillary AP deficiency.
During the consultation, the surgeon should elicit the patient’s concerns and expectations, emphasising the importance of what can be realistically achieved with malar implants. For example, patients with a maxillary hypoplasia (specifically maxillary anteroposterior (AP) deficiency) will have a class 3 malocclusion and therefore require an orthognathic procedure such as a Le Fort 1 osteotomy in addition to the placement of malar implants (Figure 3).

Figure 3: Frontal, oblique and lateral views showcasing the patient in Figure 2 who underwent placement of malar implants and a Le Fort 1 maxillary advancement osteotomy to correct his midfacial volume discrepancy. Postoperatively, he now has a convex facial profile and rejuvenated ‘youthful’ midface.
Importantly, any pre-existing deficits in sensation and facial animation from potential previous aesthetic / corrective procedures must be documented. There are few absolute contraindications to the placement of malar implants including a history of allergy to the implant material, active periodontal and / or maxillary sinus infections. It is worth noting that patients should be encouraged to give up smoking prior to the placement of malar implants as the effects of the nicotine and heat may theoretically contribute to postoperative complications of wound infection, dehiscence and eventual implant loss [1].
Implant placement
There are several approaches to placing malar implants which can either be inserted extraorally (transconjunctival or subcilliary approach) or intraorally. It is our preference to use the intraoral approach and perform the procedure under general anaesthesia with or without other aesthetic or orthognathic procedures. The patient is prepped and draped in the standard manner for a facial aesthetic procedure, with the entire face visible. We routinely inject 2% lidocaine with 1:80,000 adrenaline into the maxillary vestibule prior to making a 1cm incision commencing just above the maxillary canine root. Dissection then proceeds in the subperiosteal plane, taking care to avoid aggressive over-dissection of the midface as this may lead to inadvertent damage to the infraorbital nerve bundle, herniation of the buccal fat pad and / or an enlarged subperiosteal pocket that promotes implant mobility. The dissected subperiosteal pocket should be slightly larger than the malar implant to aid ease of insertion.
After the subperiosteal pocket is created, haemostasis is checked for and controlled prior to insertion of the implant which has been soaked in a gentamicin solution. The actual size of Medpore implant chosen is dependent upon the amount of desired malar projection and the size of the patient. The malar implant should sit passively on the maxilla and can be trimmed with scissors if needed to get the desired optimal fit. Anatomically, the ideal position of the malar implant is positioned on the maxilla with the inferior orbital rim superiorly, the zygomatic arch laterally and the piriform fossa medially (Figure 4).
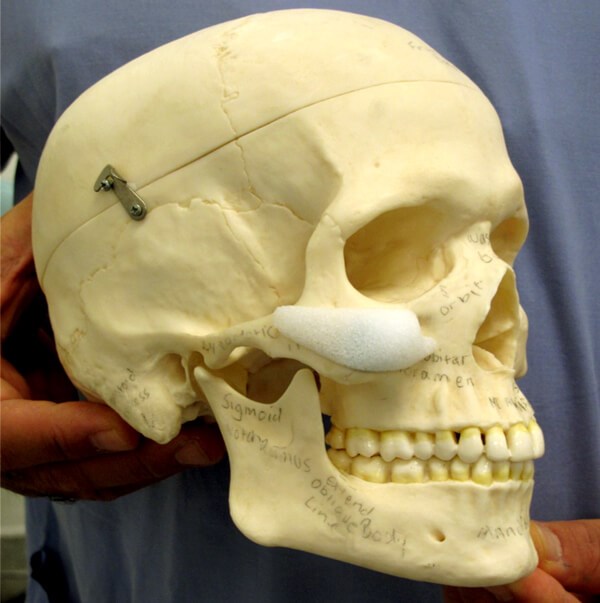
Figure 4: Diagram to highlight the optimal position of a malar implant, noted anteriorly
is the notch created adjacent to the infraorbital nerve bundle.
Theoretically, although a well-conforming implant in a tight pocket does not need to be secured, we routinely use a single fixation screw to minimise any chances of macro-movement and / or underlying bone resorption. On occasion, the tail end of the malar implant may ‘wing out’ and can be clinically palpable and / or visible over the zygomatic arch. Under these circumstances we place a second fixation screw to hold the tail down. Finally, the incision is closed with interrupted Vicryl Rapide sutures.
Postoperative protocol
There is a variable amount of oedema in the immediate postoperative period, however, no external dressings are required. Significant severe swelling may indicate an underlying haematoma, which must be drained at the earliest opportunity. This can easily be achieved by partially or fully opening the incision as indicated and evacuating the haematoma with minimal compromise to the outcome. Our patients are instructed to adhere to scrupulous oral hygiene including the use of chlorhexidine gluconate mouthwash, analgesia as required (an NSAID such as ibuprofen) and a week’s course of postoperative prophylactic antibiotics (amoxicillin and metronidazole). We recommend that the mouthwash be used as an adjunct to regular routine toothbrushing for up to seven days (twice daily). We also advise that our patients adhere to a soft diet for two weeks in order to minimise the risk of wound dehiscence and subsequent implant exposure.
“Anatomically, the ideal position of the malar implant is positioned on the maxilla with the inferior orbital rim superiorly, the zygomatic arch laterally and the piriform fossa medially”
Complications
Numerous complications associated with malar implants have been described in the literature [1,6,7], however, in our practice these are exceedingly rare and generally the implants are well tolerated. Although the intraoral approach risks bacterial contamination of the implant by oral organisms, infection rates are low. Low-grade infections can often be salvaged with a course of antibiotics. However, in cases of resistant infection the implant must be removed immediately and not replaced for minimum of six to eight weeks to allow for complete resolution of infection and inflammation. Other risks associated with malar implants include malposition / migration, underlying bone resorption and neuropraxia secondary to impingement of the implant on the infraorbital nerve.

Figure 5: (Top) Frontal, oblique and lateral views showcasing this patient who clinically demonstrates zygomatic insufficiency, an acute nasolabial angle, an under supported upper lip, over supported lower lip and lower facial asymmetry. (Bottom) This patient underwent a concomitant Le Fort 1 maxillary advancement osteotomy with bilateral malar implant placement. The before and after photos nicely illustrate the need for malar augmentation when rejuvenating the midface. The changes of the maxillary advancement are seen at the level of the dentition only, i.e. the osteotomy cut is designed and carried out in such a way that the effects of the advancement affect mainly the dentition alone. Likewise, the effects of the malar hypoplasia, lack of projection are clearly seen in the pre and postoperative photos as the implants are seated onto the area of deficit. Any change noted in this area is a true change secondary to the augmenting / volumisation effects of the implants.
Conclusion
Over the years, there have been considerable advances in midfacial implant technology [1,6], which are now designed to provide a natural-looking augmentation of the malar complex. For experienced surgeons, particularly those with an oral and maxillofacial surgical background, placement of malar implants is a simple and straightforward surgical procedure. Despite their longevity, should complications occur with placement or the patient is not happy with the final aesthetic outcome, malar implants can be easily removed. In our humble opinion, malar implants provide a robust choice for strategically addressing volume loss in the deeper structural components of the midface such as the skeletal framework (Figure 5).
References
1. Niamtu J. Essentials of cheek & midface implants. Journal of Oral & Maxillofacial Surgery 2010;68:1420-9.
2. Mendelson B, Wong CH. Changes in the facial skeleton with aging: Implications and clinical applications in facial rejuvenation. Aesthetic Plastic Surgery 2012;36(4):753-60.
3. Fitzgerald R, Graivier MH, Kane M, et al. Update on facial aging. Aesthetic Surgery Journal 2010;30(Suppl):11S‑24S.
4. Shaw RB, Katzel EB, Koltz PF, et al. Facial bone density: Effects of aging and impact on facial rejuvenation. Aesthetic Surgery Journal 2012;32(8):937-42.
5. Coleman S, Saboeiro A, Sengelmann R. A comparison of lipoatrophy and aging: Volume deficits in the face. Aesthetic Plastic Surgery 2009;33(1):14-21.
6. Yaremchuk MJ. Chapter 86. Augmentation of the facial skeleton. In: Plastics Secrets Plus. 2nd Edition. Mosby Elsevier; 2010: 564-7.
7. Sarkarat F, et al. Chapter 24. Facial augmentation with implants. In: A Textbook of Advanced Oral & Maxillofacial Surgery. Volume 2. E-book by InTech. 2015;549-68.